Tristem Medika Indonesia cell processing facility is in compliance with the standard of Ministry of Health of Indonesia.
Manufacturing and Support System

Aseptic Manufacturing (Cell Processing)
Tristem manufacturing zone follows GMP standards for aseptic processing. Manufacturing zone equipped with Biosafety Cabinets, Cell culture Incubators, and other instruments for cell processing and culture.
Tristem regularly conducts re-qualification for its manufacturing zones to ensure they still conform to GMP specifications. All cell processing equipments are periodically calibrated.
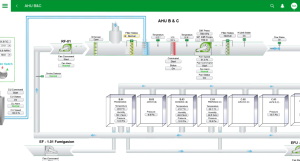
HVAC System
Tristem manufacturing facility has two independent HVAC units to maintain the temperature and humidity. One for the cell processing zone and another for QC and warehouse zones. The dedicated and separate HVAC unit allows virtually no risk of cross-contamination between cleanroom zones. The HVAC system is equipped with real-time monitoring and logging system 24/7 to monitor pressure differentials, temperature, and humidity in each room. HVAC sensors are regularly calibrated.

Cryopreservation
Processed cells are cryopreserved and stored in the vapor phase of liquid nitrogen(LN2) storage vessels. The vapor phase storage minimizes the contamination risk.
