Mesenchymal Stem Cell
Stem Cell Therapy is a regenerative therapy to repair damaged tissues and organs. The stem cell is like spare parts in our body that repair damaged tissues and return tissue and organs to a healthier state.
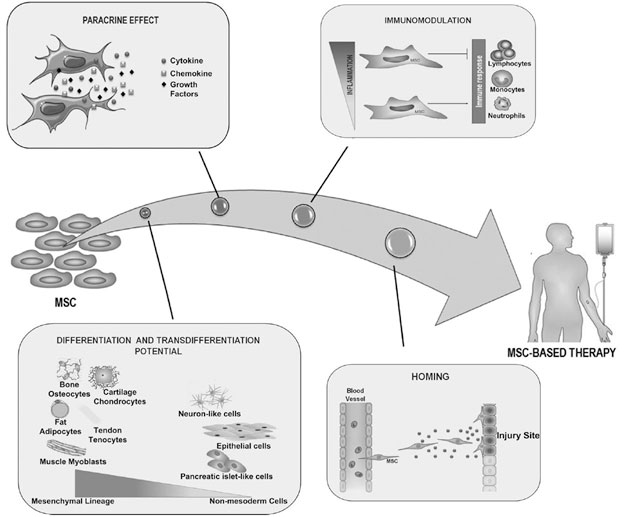
Mesenchymal Stem Cells (MSCs) are a form of adult stem cell that has the ability to repair and renew damaged tissue. The MSCs move to the injury site (homing) and then release various cytokine, chemokine, and growth factors to reduce inflammation and promote tissue repair, as well as inducing the indigenous stem cell to proliferate and differentiate to replace damaged cells.
Tristem processes the MSC using proprietary technology that is able to replicate the stem cell natural environment “micro-niche” where the cell originates; thus, the stem cell’s “stemness” properties are better preserved, highly proliferative, produce more growth factors, and minimal risk of rejection (almost zero HLA-DR).
In addition, we only use GMP grade medium and supplement without serum and animal origin (xeno-free). The culture processes following the isolation phase are free from antibiotics, minimizing the risk of allergic reaction and ensuring the highest cell quality.
Stem Cell Therapy and its derivative is an experimental treatment. Almost no stem cell therapy has been approved worldwide since the approval for a new form of treatment is a time-consuming process. Thus, this therapy is restricted to the facility with legal permission. Tristem has been licensed since 2020 by the Ministry of Health Republic of Indonesia to process stem cell and its derivative.